Sutures Needles
Category
NEED MORE
INFORMATION
CONTACT US
Biotronix Healthcare Customer Service Associates are available 8:30am to 5:pm EST Monday thru Friday
North America Customer Service
Tel: (954) 320-6088
(954) 440-1572
customerservice@biotronixhealthcare.com
International Customer Service
Tel: (289) 813-0159
(416) 876-5035
customerservice@biotronixhealthcare.com
______________________________________
Sales and Orders
Tel: (954) 266-8944
sales@biotronixhealthcare.com
______________________________________
Regulatory Affairs
regulatory@biotronixhealthcare.com
______________________________________
Accounting Department
accounting@biotronixhealthcare.com
______________________________________
Call to speak with a knowledgeable Biotronix Healthcare specialist today.

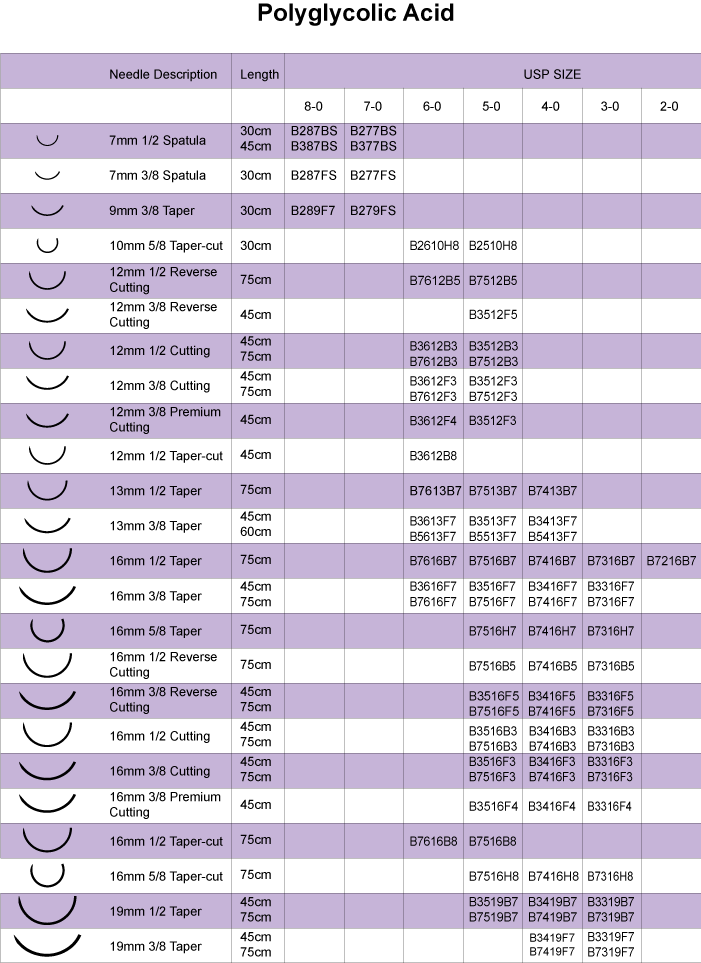
Polyglycolic Acid
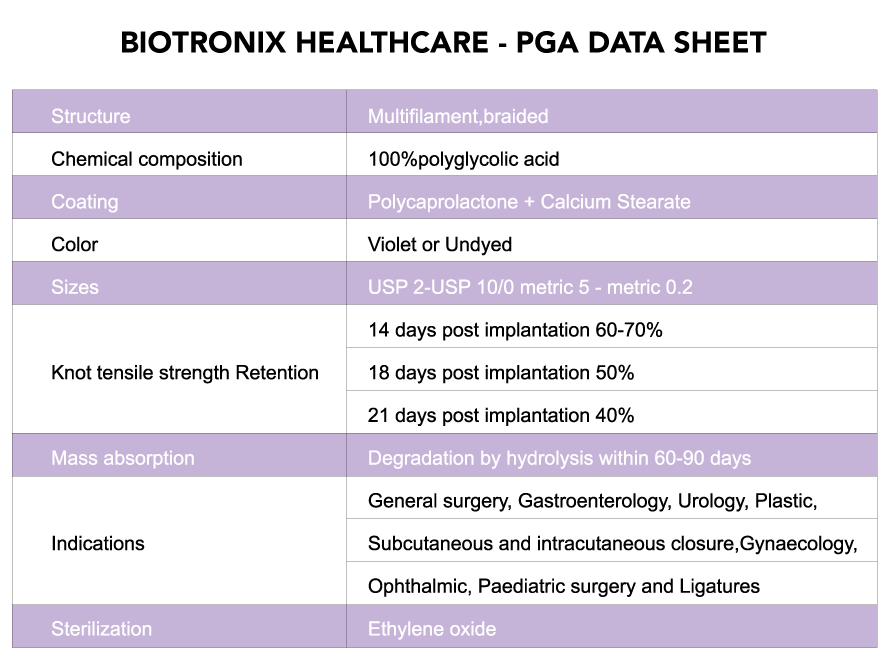
Biotronix Healthcare Polyglycolic Acid sutures are synthetic absorbable sutures composed of polyglycolic acid and a calcium stearate and polycaprolactone coating.
Biotronix Healthcare Polyglycolic Acid sutures has minimal tissue reactivity during the absorption process, superior tensile strength and excellent knotting security. The braided construction ensures that handling and knotting are excellent and the unique lubricant coating remains effectively bonded to the material throughout its use, ensuring smooth passage through tissue and easy knot tie down.
Biotronix Polyglycolic Acid sutures hold more than 75% of its tensile strength at two weeks and complete absorption takes place in approximately 60 to 90 days.
Applications:
 Biotronix’ Polyglycolic Acid sutures are ideal for use in general surgery, orthopedic surgery, obstetric/gynecological surgery, gastro intestinal tract.
Biotronix’ Polyglycolic Acid sutures are ideal for use in general surgery, orthopedic surgery, obstetric/gynecological surgery, gastro intestinal tract.
Characteristics of Biotronix Healthcare Polyglycolic Acid sutures:
• Drill End Press Fit needles for maximum strength between thread and needles
• Unique lubricant coated ensures smoother passage through tissue
• High tensile strength
• Superb Handling properties
• Minimal tissue irritation
• Secure knot placement
• Maintains more than 75% of tensile strength at 14 days
• Maintains 50% tensile strength at 21 days
• Complete absorption in 60 to 90 days from implantation
Packaging:
Individually packaged 12 or 36 per box. Also available in reels.
Contraindications
This suture, being absorbable, should not be used where extended approximation of tissue is required. The use of this suture may be inappropriate in elderly, malnourished, or debilitated patients, or in patients suffering from conditions which may delay wound healing. Not for use in cardiovascular and neurological surgery.
Warnings
Do not use if package is open or damaged or if the expiration date has been exceeded. Discard open, unused suture. Do not re-sterilize. Re-sterilization may alter the physical properties Users should exercise caution when handling surgical needles to avoid inadvertent needle sticks. Discard used needles in a “sharps” container.
Avoid storing product at elevated temperatures. As with any foreign body, prolonged contact of this or any other suture with salt solutions, such as those found in the urinary or biliary tracts, may result in calculus formation. Acceptable surgical practice should be followed with respect to drainage and closure of contaminated or infected wounds.
The use of supplemental non-absorbable sutures should be considered by the surgeon in the closure of sites which may undergo expansion, stretching, or distention, or which may require additional support as this is an absorbable suture material.
Precautions
Skin sutures, which must remain in place longer than 7 days may cause localized irritation and should be snipped off or removed as indicated. In handling this or any other suture material, care should be taken to avoid damage from handling.
Avoid crushing or crimping damage due to application of surgical instruments such as forceps or needle holders.
Adequate knot security requires the accepted surgical technique of flat, square ties, with additional throws as warranted by surgical circumstances and the experience of the surgeon. Users should be familiar with surgical procedures and techniques involving absorbable suture before employing Polyglycolic Acid
synthetic absorbable suture for wound closure, as risk of wound dehiscence may vary with the site of application and the suture material used.
Adverse reactions
Adverse effects associated with the use of this device include wound dehiscence, failure to provide adequate wound support in closure of the sites where expansion, stretching or distension occur, failure to provide adequate wound support in elderly, malnourished or debilitated patients or in patients suffering from conditions which may delay wound healing, wound infection, minimal acute inflammatory tissue reaction, localized irritation when skin sutures are left in place for greater than 7 days, calculi formation in urinary and biliary tracts when prolonged contact with salt solutions such as urine and bile occurs, and transitory local irritation.