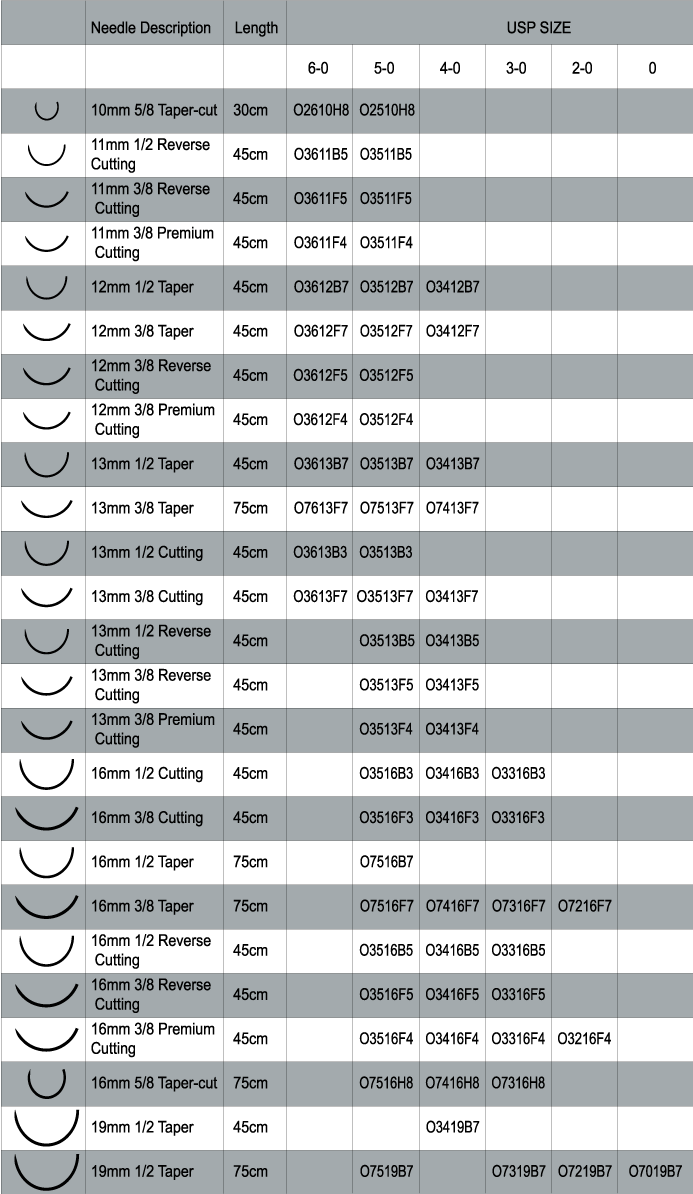
Sutures Needles
Category
NEED MORE
INFORMATION
CONTACT US
Biotronix Healthcare Customer Service Associates are available 8:30am to 5:pm EST Monday thru Friday
North America Customer Service
Tel: (954) 320-6088
(954) 440-1572
customerservice@biotronixhealthcare.com
International Customer Service
Tel: (289) 813-0159
(416) 876-5035
customerservice@biotronixhealthcare.com
______________________________________
Sales and Orders
Tel: (954) 266-8944
sales@biotronixhealthcare.com
______________________________________
Regulatory Affairs
regulatory@biotronixhealthcare.com
______________________________________
Accounting Department
accounting@biotronixhealthcare.com
______________________________________
Call to speak with a knowledgeable Biotronix Healthcare specialist today.
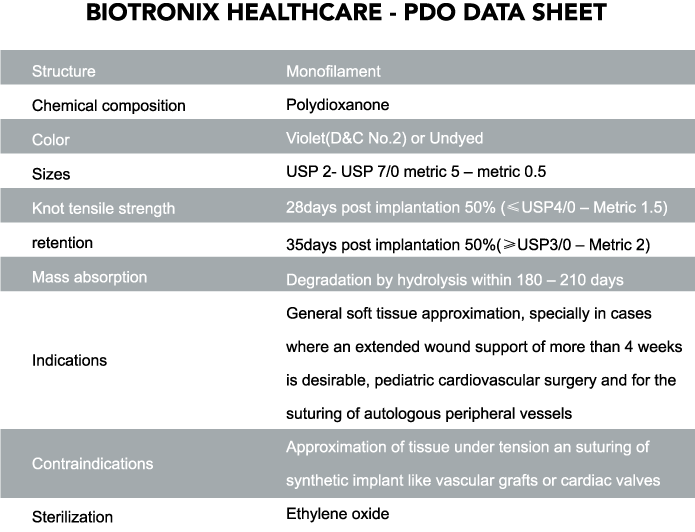
Polydioxanone
Biotronix Healthcare Polydioxanone suture is a synthetic absorbable sutures composed of polyester, poly (p-dioxanone). Biotronix Healthcare Polydioxanone sutures have minimal tissue reactivity during the absorption process, superior tensile strength and excellent knotting security.
Biotronix Polydioxanone sutures holds more than 80% of its tensile strength at two weeks and complete absorption at 180 days.
Applications:
 Polydioxanone suture is ideal for use in general surgery, orthopedic surgery, pediatric cardiovascular surgery, gastro intestinal tract and sub cuticle surgery.
Polydioxanone suture is ideal for use in general surgery, orthopedic surgery, pediatric cardiovascular surgery, gastro intestinal tract and sub cuticle surgery.
Characteristics of Biotronix Healthcare Polydioxanone suture:
• Drill End Press Fit needles for maximum strength between thread and needles
• Minimal tissue reactivity
• High tensile strength
• Maintains 80% of tensile strength at 14 days
• Maintains 40% tensile strength at 60 days
• Complete absorption in 180 days from implantation
Packaging:
Individually packaged 12 or 36 per box. Also available in reels.
Contraindications
These sutures, being absorbable, are not to be used where prolonged (beyond six weeks) approximation of tissues under stress is required and are not to be used in conjunction with prosthetic devices, i.e., heart valves or synthetic grafts. Polydioxanone suture is not indicated in adult cardiovascular tissue, microsurgery and neural tissue.
Warnings
The safety and effectiveness of Polydioxanone suture has not been established in neural tissues, adult cardiovascular tissue or for use in microsurgery. Under certain circumstances, notably orthopaedic procedures, immobilization by external support may be employed at the
Do not use if package is open or damaged or the expiration date has been exceeded. Discard open, unused suture. Do not resterilize; resterilization may alter the physical properties of this suture.
Precautions
Polydioxanone suture knots must be properly placed to be secure. As with other synthetic sutures, knot security requires the standard surgical technique of flat and square ties with additional throws if indicated by surgical circumstances and the experience of the operator.
As with any suture, care should be taken to avoid damage when handling. Avoid the crushing or crimping application of surgical instruments, such as needle holders and forceps, to the strand except when grasping the free end of the suture during an instrument tie. Conjunctival and vaginal mucosal sutures remaining in place for extended periods may be associated with localized irritation and should be removed as indicated.
Subcuticular sutures should be placed as deeply as possible in order to minimize the erythema and induration normally associated with absorption. Acceptable surgical practice should be followed with respect to drainage and closure of infected wounds.