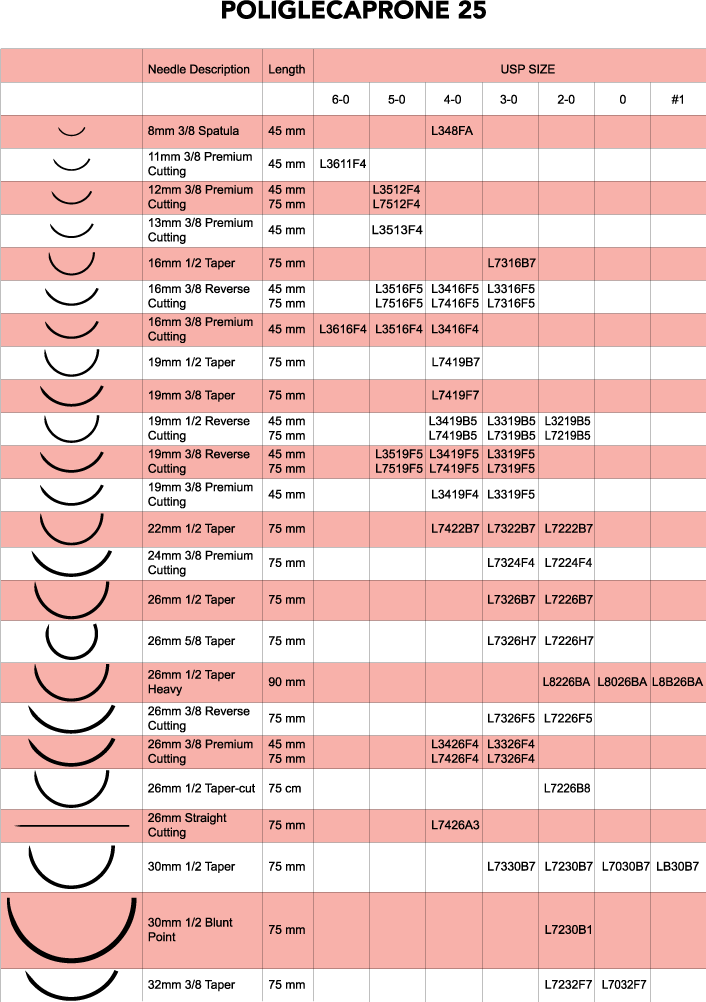
Sutures Needles
Category
NEED MORE
INFORMATION
CONTACT US
Biotronix Healthcare Customer Service Associates are available 8:30am to 5:pm EST Monday thru Friday
North America Customer Service
Tel: (954) 320-6088
(954) 440-1572
customerservice@biotronixhealthcare.com
International Customer Service
Tel: (289) 813-0159
(416) 876-5035
customerservice@biotronixhealthcare.com
______________________________________
Sales and Orders
Tel: (954) 266-8944
sales@biotronixhealthcare.com
______________________________________
Regulatory Affairs
regulatory@biotronixhealthcare.com
______________________________________
Accounting Department
accounting@biotronixhealthcare.com
______________________________________
Call to speak with a knowledgeable Biotronix Healthcare specialist today.
Poliglecaprone 25
Biotronix Healthcare Poliglecaprone 25 sutures are synthetic absorbable sutures composed of a copolymer of glycolide and epsilon-caprolactone.
Biotronix Healthcare Poliglecaprone 25 sutures has minimal tissue reactivity during the absorption process, superior tensile strength and excellent knotting security. Biotronix Poliglecaprone 25 sutures are a reliable rapid absorbtion suture.
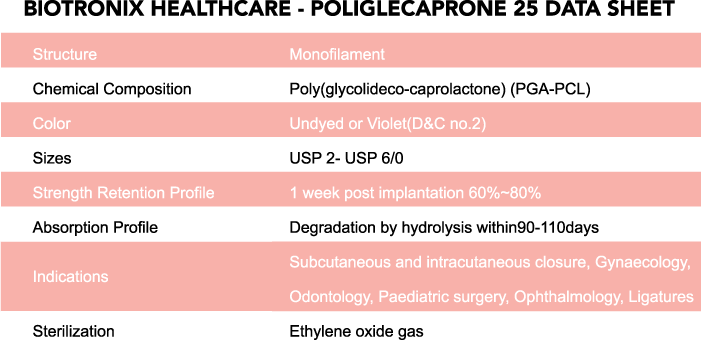
 Applications:
Applications:
Poliglecaprone 25 sutures are ideal for use in soft tissue approximation and or ligation, but for use in cardiovascular, neurological procedures microsurgery and ophthalmic surgery.
Characteristics of Biotronix Healthcare Poliglecaprone 25 sutures:
• Smooth tissue passage
• Minimal tissue reactivity
• Excelent handling properties
• High pliability
• Complete absorption in 90 to 120 days from implantation
Packaging:
Individually packaged 12 or 36 per box.
Contraindications
This suture, being absorbable, should not be used where extended approximation of tissue is required. The use of this suture may be inappropriate in elderly, malnourished, and debilitated patients, or in patients suffering from conditions that may delay wound healing. Not for use in cardiovascular or neurological surgery, microsurgery, or ophthalmic surgery.
Warnings
Do not resterilize; resterilization may alter the physical properties of this suture. Do not use if package is open or damaged or if the expiration date has been exceeded. Discard open, unused suture. Users should exercise caution when handling surgical needles to avoid inadvertent needle sticks. Discard used needles in a “sharps” container. Avoid storing product at elevated temperatures.
Precautions
Skin sutures which must remain in place longer than 7 days may cause localized irritation and should be snipped off or removed as indicated. Subcuticular sutures should be placed as deeply as possible to minimize the erythema and induration normally associated with absorption.
Consideration should be taken in the use of absorbable sutures in tissue with poor blood supply as suture extrusion and delayed absorption may occur. In handling this or any other suture material, care should be taken to avoid damage from handling. Avoid crushing or crimping damage due to the application of surgical instruments such as forceps or needle holders.
PGCL suture knots must be properly placed to be secure. Adequate knot security requires the accepted surgical technique of flat, square ties, with additional throws as warranted by surgical circumstance and the experience of the surgeon. The use of additional throws may be particularly appropriate when tying monofilaments. As with any foreign body, prolonged contact of this or any other suture with salt solutions, such as those found in the urinary or biliary tracts, may result in calculus formation. As an absorbable suture, PGCL suture may act transiently as a foreign body. Acceptable surgical procedure must be followed with respect to drainage and closure of contaminated or infected wounds. Users should be familiar with surgical procedures and techniques involving absorbable sutures before employing PGCL sutures for wound closure, as the risk of wound dehiscence may vary with the site of application and the suture material used.
Adverse reactions
Adverse effects associated with the use of this device include wound dehiscence, failure to provide adequate wound support in sites where expansion, stretching, or distention occur, failure to provide adequate wound support in elderly, malnourished, and debilitated patients or in patients suffering from conditions that may delay wound healing, infection, minimal acute inflammatory tissue reaction, localized irritation when skin sutures are left in place for greater than 7 days, suture extrusion and delayed absorption in tissue with poor blood supply, calculi formation when prolonged contact with salt solution occurs, enhanced bacterial infectivity, and transitory local irritation.