Sutures Needles
Category
NEED MORE
INFORMATION
CONTACT US
Biotronix Healthcare Customer Service Associates are available 8:30am to 5:pm EST Monday thru Friday
North America Customer Service
Tel: (954) 320-6088
(954) 440-1572
customerservice@biotronixhealthcare.com
International Customer Service
Tel: (289) 813-0159
(416) 876-5035
customerservice@biotronixhealthcare.com
______________________________________
Sales and Orders
Tel: (954) 266-8944
sales@biotronixhealthcare.com
______________________________________
Regulatory Affairs
regulatory@biotronixhealthcare.com
______________________________________
Accounting Department
accounting@biotronixhealthcare.com
______________________________________
Call to speak with a knowledgeable Biotronix Healthcare specialist today.

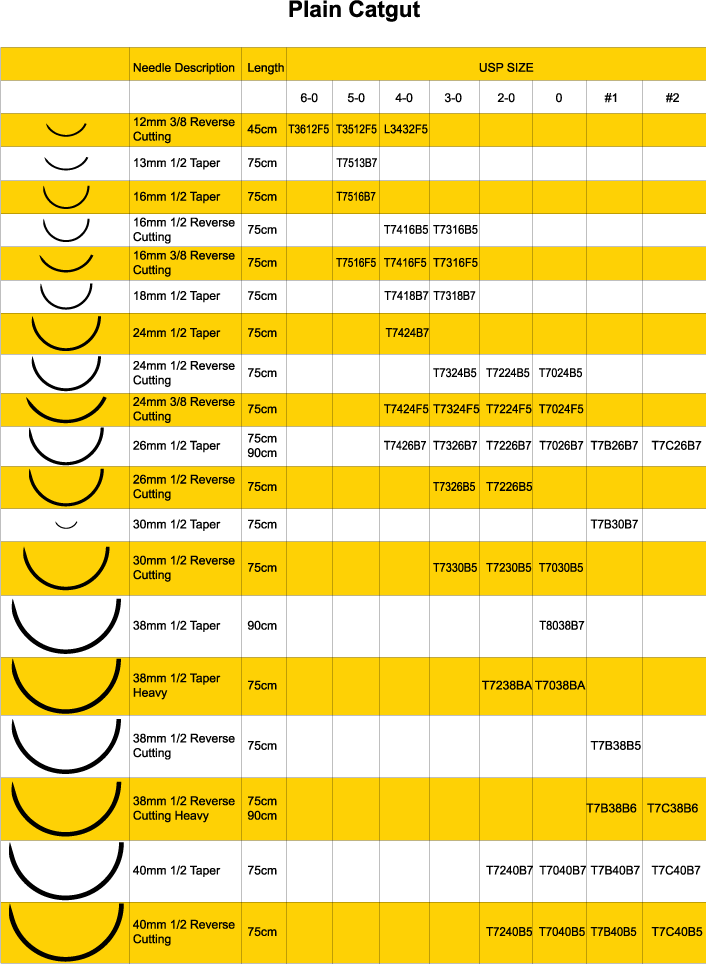
Plain Catgut
Biotronix Healthcare plain catgut suture are natural absorbable twisted monofilament sutures composed of highly purified connective tissue from both, the serosal layer of bovine intestine or the intestinal sub-mucosa layer of sheep. Biotronix Healthcare plain catgut has minimal tissue reactivity and a short absorption period. Biotronix plain catgut suture is of the highest quality with collagen compositions of over 95% ensuring higher tensile strength and absorption times. Biotronix plain catgut suture holds tensile strength on implantation from 12-15 days and complete absorption takes place in approximately 30-60 days.

Applications:
Biotronix Plain Catgut suture is ideal for use in general soft tissue approximation and/or ligation, including use in ophthalmic procedures.
Characteristics of Biotronix Healthcare Plain Catgut sutures:
• Drill End Press Fit needles for maximum strength between thread and needles
• Accurate polishing ensures smoother passage through tissue
• High collagen purity
• Superb Handling properties
• Minimal tissue irritation
• Uniform twist spread across the suture length and controlled dying provides high tensile strength
• Absorption by enzymatic hydrolysis
• Complete absorption in 30 to 60 days from implantation
Packaging:
Individually packaged 12 or 36 per box. Also available in reels.
Contraindication
The use of this sutures is contraindicated in patients with known sensitivities or allergies to any of its components. These sutures, being absorbable, should not be used where extended approximation of tissue is required.
Do Not resterilize. Discard open, unused sutures. Store at room temperature. Avoid prolonged exposure to elevated temperatures.
Prolonged contact of this or any other sutures with salt solutions, such as those found in the urinary or biliary tracts, may result in calculus formation.
Users should be familiar with surgical procedures and techniques involving gut suture before using Plain and Chromic Surgical gut sutures for wound closure, as the risk of wound dehiscence may vary with the site of application and the suture material used. In cases where closure of site may undergo expansion, stretching, or distention, the use of supplemental nonabsorbable sutures should be considered.
Accepted surgical practices must be followed with the respect to the management of contaminated or infected wounds. Also use of these sutures is not appropriate in elderly, debilitated, malnourished, or patients with conditions which may delay wound healing. Certain patients may be hypersensitive to collagen or chromium and might display an immunological reaction resulting in inflammation, fibrosis, wound suppuration and bleeding as well as sinus formation
Precautions
In handling this or any other suture material, care should be taken to avoid damage from handling. Avoid crushing or crimping damage due to application of surgical instruments such as forceps or needle holders.
The surgeon should avoid unnecessary tension when running down knots, to reduce the occurrence of surface fraying and weakening of the strand. Adequate knot security requires flat, square ties with additional throws as warranted by surgical circumstances and the experience of the surgeon.
Adverse reactions
Adverse effects associated with the use of this device include: wound dehiscence, increased rates of absorption over time (depending on the type of suture used, the presence of infection and the tissue site), failure to provide adequate wound support in closure of the abdomen, chest joints, and other sites where failure to provide adequate wound support in elderly, malnourished or debilitated patients or in patients suffering from cancer, anemia, obesity, diabetes, infection or other conditions which may delay wound healing, allergic response in patients with known sensitivities to collagen or chromium which may result in an immunological reaction resulting in inflammation, tissue granulation or fibrosis, wound suppuration and bleeding, as well as sinus formation, enhanced bacterial infectivity, moderate tissue inflammatory response characteristic of foreign body response, calculi formation in urinary and biliary tracts when prolonged contact with salt solutions such as urine and and bile occurs, and pain edema and erythema at the wound site.